NECTAR at a glance
Coordinator:
Pavia University (UNIPV) Italy
Budget:
3.2 million euro
Duration:
01.04.2021 - 30.09.2025
ALZHEIMER DISEASE (AD) IS THE MOST COMMON CAUSE OF DEMENTIA BUT NO CURE OR EFFECTIVE TREATMENTS EXIST.
The majority of researched treatments are based on drug development program targeting the beta amyloid peptide (Aβ). The Aβ is considered to be the main culprit of the neurodegenerative process, characterized by progressive aggregation: oligomers (spheric aggregates with diameters of few nm) develop into fibrils (“chains” of oligomers of hundreds μm length and few nm diameter) up to plaques of tens μm diameter, that cause the irreversible decline of synaptic and cognitive functions.
NECTAR PROJECT PROPOSES AN ALTERNATIVE AND REVOLUTIONARY STRATEGY TO ADDRESS ALZHEIMER DISEASE, INVESTIGATING A THERAPY BASED ON IONIZING RADIATIONS.
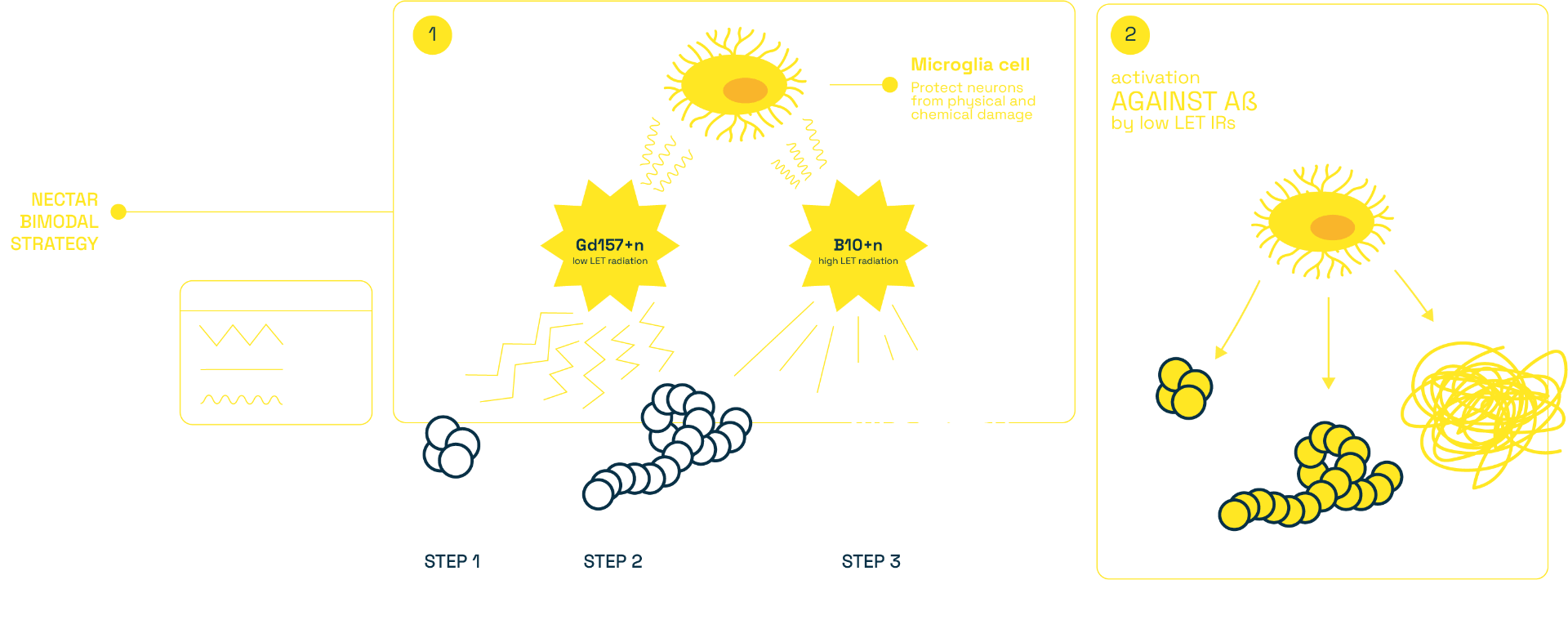
NECTAR aims to prove the feasibility, safety and effectiveness of a Capture-Enhanced Neutron Irradiation (CENI) to structurally damage the Abeta aggregates: it exploits an external beam of low energy neutrons which selectively targets the amyloid beta aggregates through specifically engineered radiation enhancers, containing Boron-10 and Gadolinium-157 as capture agents. The nuclear capture reactions, induced by the neutron irradiation, generate a mixed high+low LET radiation field, expected to induce a bimodal treatment: (i) a local depolymerisation of Aβ aggregates by the highly biological effective charged particles and (ii) a long distance stimulation of the brain tissue by penetrating photons.
Work Packages
Lead beneficiary: Pavia University, UNIPV
Involved partners: UNITO, IRFMN, Raylab, IRSN,
SU, UKESSEN, UKW
Objectives: to ensure the operational management of NECTAR project
by:
1. organization of project meetings,
2. managing the Steering Committee,
appointing and managing the Scientific Advisory Board (SAB),
3. piloting the project
reporting activity.
Lead beneficiary: Torino University, UNITO
Involved partners: IRFMN, IRSN,
UKESSEN
Objectives:
1. synthesis of biocompatible probes based on Gd/B-enriched
molecules able to (i) pass the health Blood-Brain Barrier (BBB) and (ii) selectively bind
Aβ aggregates,
2. characterization of the probes (stability, binding affinity to
Aβ protein and aggregates, spatial distribution of capture-enhanced nuclides around and
inside the Aβ aggregates),
3. in vivo biodistribution.
Lead beneficiary: Institut de Radioprotection et de Sûreté Nucléaire,
IRSN
Involved partners: UNIPV, UNITO, IRFMN, Raylab, SU, UKW
Objectives:
1.
to develop computational tools to predict and correlate the actions of ionizing radiations
set in motion by CENI treatment to the effects (structural and biological) observed in
post-irradiated Abeta aggregates;
2. to design proper set-ups for the irradiation of
protein water solution, small animals and AD patients.
Lead beneficiary: Raylab s.r.l., Raylab
Involved partners: UNIPV, UNITO, IRFMN, Raylab,
IRSN, SU, UKESSEN
Objectives:
1. to ascertain the capability of Capture-Enhanced
Neutron Irradiation (CENI) to induce structural depolymerization of Abeta species marked by
Gd/B in a cell-free approach;
2. to study the residual neurotoxic effects of such
aggregates in vitro and in vivo;
3. to perform micro- and nano-dosimetry measurements
to compare with the computational tools developed in WP3.
Lead beneficiary: Stockholm University, SU
Involved partners: UNIPV, UNITO, IRFMN,
Raylab, UKESSEN, UKW
Objectives:
1. to define the cellular and cytogenetic effects
of low energy neutron irradiation alone and in combination with Gd/B enhancement agents on
neuronal and glia cells;
2. to identify eventual toxic effects of low energy neutrons
irradiation alone and in combination with Gd/B enhancement agents in the brain of C57 naive
and APP/PS1 AD mice.
Lead beneficiary: Mario Negri Institute for Pharmacological Research IRCCS,
IRFMN
Involved partners: UNIPV, UNITO, Raylab, SU, UKESSEN
Objectives:
1. to
test the therapeutic efficacy of CENI-mediated Gd/B treatment to restore memory in the
APP/PS1 AD mice at both pre- and post-symptomatic stages;
2. to test the therapeutic
efficacy of Gd/B to reduce the extent of neuropathology at the level of multiple
neuropathological targets in APP/PS1 AD mice at both pre- and post-symptomatic stages.
Lead beneficiary: Pavia University, UNIPV
Involved partners: UNITO, IRFMN, Raylab, IRSN,
SU, UKESSEN, UKW
Objectives:
1. to disseminate project results to the different
audiences impacted by the research,
2. to ensure the internal and external
communication around the project,
3. to train end-users of the innovative AD treatment
strategy.
Work Packages in Years
1
2
3
4

NECTAR scientific background
The effectiveness of External Beam Radiation Therapy (EBRT) in the treatment of localized
TracheoBronchial Amyloidosis (TBA) is known[1-3] such that photon RT is presently
recommended as a first-line treatment for patients who are not eligible for
bronchoscopic interventions[4]. The total dose is generally 20Gy, 2Gy/fraction in 2
weeks. All the patients showed improvement or stabilisation of the symptoms with mild
and manageable side effects.
The mechanisms by which RT is effective on TBA is
still unclear. Several hypotheses have been proposed, although none clearly supported by
clinical data. The most discussed one is that RT acts on local plasma cells which are
considered the culprits of the amyloid deposits due to the up regulated secretion of
peptide light chains. However, the analysis of amyloid deposits showed scarce presence
of plasma cells thus a radiation effect on their function or turnover is unlikely to be
the only mechanism in action.
Taking into account the common amyloidosis nature of
TBA and AD, the rationale for a radiation induced depolymerisation of Aβ aggregates
was suggested[5]. The capability of IRs to change amyloid structure by depolymerise
associated scaffold molecules (glucosaminoglycans, GAGs, invariably associated with
amyloid fibrils) is a DNA-independent mechanism, thus a regimen based on very low dose
per fraction over a prolonged treatment period can match the brain tolerance levels and
still slows the cognitive impair.
Bibliography:
[1] Kalra et al, Mayo Clin Proc 2006 76:853-856;
[2] Kurrus et
al, Hest 1998 114:1489-1492;
[3] Monroe et al, Hest 2004 125:784-789;
[4]
Neben- Wittich et al, Chest 2007 132:262-267;
[5] Bistolfi, Neuroradiol J 2008
21:683-692.
Compared to the literature available at NECTAR project submission and start, the papers testing preclinically the effectiveness of conventional radiotherapy in the treatment of AD are steadily increasing. Considering the earliest one [1] which evaluated the effects of X-rays RT on in vitro preformed Aβ1-42 fibrils and on their initial formation using the Thioflavin-T assay and acute regimes of radiations (single fractions from 5 up to 20Gy) with poor significant effects by the irradiation, nowadays the papers studying the effects of photons in murine models of AD are several [2-5].
Despite further investigations are needed, the expression of genes and promoting factors connected with the activation of the brain immune system is the dominant hypothesis supporting that low dose and low dose rate of conventional irradiation can stimulate the clearance of the Aβ aggregates by the recruitment of glia cells.
Bibliography:
[1] Patrias et al, Radiar Res 2011 175:375-381; [2] Marples et al,
Radiother Oncol 2016 118:43-
51; [3] Kim et al, Int J Mol Sci 2020, 21, 3678; [4]
Kim et al, Int J Mol Sci 2020, 21, 4532 ; [5]
Wilson et al, J Alzheimers Dis 2020,
75(1):15-21.
A case report[1] described for the first time the remarkable improvement observed in a patient with advanced AD who received 5 CT-scans of the brain (about 40 mGy each) over a period of 3 months.
The underling mechanism appears to be the radiation-induced up-regulation of the
patient’s adaptive protection systems[2], which partially restored cognition,
memory, speech, movement and appetite. More recently the same research group tested
these results in a single-arm pilot clinical trial to examine through a more
quantitative approach the effects of low-dose ionizing radiations (LDIR) in severe
AD[3]. Minor improvements on quantitative measures were noted in 75% of patients,
despite the qualitative observations of cognition and behaviour
suggested
remarkable improvements within days post-treatment, including greater overall alertness.
In the wake of these encouraging results, other trials have been initiated and are currently underway [4]. In general, nowadays the international scientific community is informed of the exciting possibilities that conventional RT can offer in the treatment of AD [5-6].
Bibliography:
[1] Cuttler et al, Dose Response 2016:1-7; [2] Feinendegen et al,
Health Phys 2011 100(3):247-259; [3] Cuttler et al, J Alzheimer's Dis 2021
80:1119-1128; [4] Rogers et al, Int J Radiat Oncol Biol Phys (article in press); [5]
Wilson and Marples, Radiat Res 185(5):443-448 (2016); [6] Wilson et al., Radiat Res
199(5): 506-516 (2023).
Links
About FET-OPEN program (now evolved in EIC Pathfinder Open)
FET (Future and Emerging Technologies) Open European program provides support for the earliest stage of new cutting-edge researches in any field of science and technologies. It is now evolved in the EIC Pathfinder Open, that continues the mission embracing high-risk, high- gain projects and interdisciplinary collaborations that drive technological breakthroughs. The program aims to go beyond existing knowledge and encourages visionary thinking to pave the way for innovations, radical new ideas and novel technologies, with potential to create new markets and opportunities.
This project has received funding from the European Union's Horizon 2020 research and innovation programme under Grant Agreement No. 964934
© 2023 Nectar Consortium. All rights reserved.